It was a fantastic week as the photos below show........












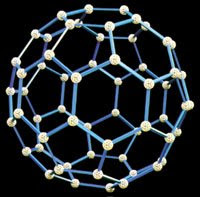 Year 9 have been researching the current applications of nanotubes and other areas of nanotechnology - the science of building machines at sub-atomic level.
Year 9 have been researching the current applications of nanotubes and other areas of nanotechnology - the science of building machines at sub-atomic level.

Scott Heng, Paul Wong, Scott Lim and Qin Yi researched the most recent applications of nano-tubes and discovered that they are 50 times stronger than steel and are currently being developed as molecular sized wires in electronic circuitry. They explained how these molecular sized nano-wires are currently being developed to produce miniature gadgets.



Mrs Kitchener




 Explanation:
Explanation:
Lots more investigating to do in the coming weeks …wonder what we will discover?
 The FIB 10 JGo class under Ms. Carter experimented with plant cells and animal cells. For plant cells, we used onion cells and human cheek cells for animal cells.
The FIB 10 JGo class under Ms. Carter experimented with plant cells and animal cells. For plant cells, we used onion cells and human cheek cells for animal cells.
Petrus Bosa Layarda
 The first meeting was held on 23rd January 08 with a group of students ranging from Grade 7 to Grade 11, who assembled together in Lab 7 under the supervision and guidance of Mrs. Kitchener.
The first meeting was held on 23rd January 08 with a group of students ranging from Grade 7 to Grade 11, who assembled together in Lab 7 under the supervision and guidance of Mrs. Kitchener.  Welcome to the SJI International Science Department blog! It is our intention to regularly post updates about the latest activities in the department to keep you informed about the fantastic classes and opportunities that SJI International science students are involved in. With regular contributions from our students we hope this blog will become an important communication tool for those interested in science in the SJI International community.
Welcome to the SJI International Science Department blog! It is our intention to regularly post updates about the latest activities in the department to keep you informed about the fantastic classes and opportunities that SJI International science students are involved in. With regular contributions from our students we hope this blog will become an important communication tool for those interested in science in the SJI International community.